- Classes of antibodies to coronavirus
- What does the coronavirus antibody test show?
- When antibodies to coronavirus are produced
- What level of neutralizing antibodies protects against COVID-19
- How many antibodies persist after infection with coronavirus
- Is the person infected with SARS-CoV-2 antibodies infected?
- Coronavirus and vaccination during pregnancy: are antibodies passed on to the baby
- Antibodies are transmitted through breastfeeding
- Neutralizing antibodies in children and adults: relationship with age and disease
- Dynamics of antibody production depending on the severity of COVID-19
- Is it possible to get infected with COVID-19 again if there are antibodies
- Vaccinated COVID-19-recovered patients have the most substantial immunity to coronavirus
- IgM Antibodies Protect Against Delta and Omicron Strains
- References
Classes of Antibodies to Coronavirus
- Immunoglobulin IgA is the first line of defense against infection. IgA is found in body secretions and protects the mucous membranes: respiratory tract, eyes, gastrointestinal tract. French scientists have shown that IgA antibodies are often detected before the appearance of IgG and IgM antibodies, with the neutralizing activity of IgA being the highest. Scientists suggest that IgA antibodies play a vital role in the early neutralization of the virus and recommend using an IgA antibody test for the early diagnosis of COVID-19. The number of IgA antibodies gradually decreases within 1 month from the onset of symptoms.
- Immunoglobulin IgM – produced first in response to SARS-CoV-2 to fight infection. If IgM antibodies appear, it means that acquired immunity has been activated. However, the IgM antibody is too large to penetrate all parts of the body. For example, IgM does not cross into the placenta. As the patient recovers, the IgM concentration drops, and they disappear.
- Immunoglobulin IgG – produced last. Only IgG antibodies provide long-term protection against the virus since they can remain in the body for several years. IgG is the superior antibodies that penetrate the placenta and provide immune protection to the baby.
Viral proteins are targets against which antibodies are produced. After contact with coronavirus, the body produces 2 types of antibodies:
- Antibodies to viral spike protein (S, anti-spike antibodies). S-antibodies show potent neutralizing activity. They are used to treat patients with severe COVID-19 and to create vaccines.
- Antibodies to viral RNA (N, antibodies to nucleocapsid). N-antibodies confirm the fact of the transferred disease but do not guarantee protection against reinfection with coronavirus.
S and N antibodies were found in infected and recovered patients with mild disease.
The body can contain many variants of antibodies at the same time – it depends on individual characteristics. How many antibodies have developed and how long they will live is also a unique feature. Only neutralizing antibodies protect against COVID-19.
What Does The Coronavirus Antibody Test Show?
The presence of antibodies to coronavirus means that a person has been infected with SARS-CoV-2 or vaccinated against COVID-19.
With COVID-19, the antibody test results can roughly estimate the stage of the disease:
- If IgM antibodies appear, then the infection is in the acute phase.
- If IgG antibodies appear, but there is still a lot of IgM, the healing process has begun.
- If only IgG is left or there is still a little IgM, then the immune system has defeated the disease and has formed an immune memory, which will protect against reinfection for a while.
When Antibodies to Coronavirus are Produced
Antibodies IgM and IgG to SARS-CoV-2 develop 6-15 days after the onset of the disease. Average time to detectable level:
- IgM + IgG (total antibodies) – 11 days after the onset of symptoms;
- IgM – 12 days;
- IgG – 14 days.
The presence of antibodies was detected in less than 40% of patients within 1 week of the onset of the disease. However, from the 15th day after the onset of the disease, the presence of antibodies rapidly increased to 100% (total antibodies), 94.3% (IgM), and 79.8% (IgG).
According to a study by an international team of scientists, 73.9% of patients produce stable neutralizing antibodies 3 weeks after the onset of the disease. Higher titers of neutralizing antibodies are found in critically ill patients.
With mild COVID-19, the detection of antibodies may take longer – 4 weeks or more. Sometimes IgM and IgG antibodies are not detected at all.
The antibody titer shows the number of immunoglobulins but not their effectiveness. On average, the more severe the course of the disease, the more effective and long-term immunity.
A balanced microbiota and dietary fiber in the diet stimulate the production of antibodies. Alcohol suppresses antibody production.
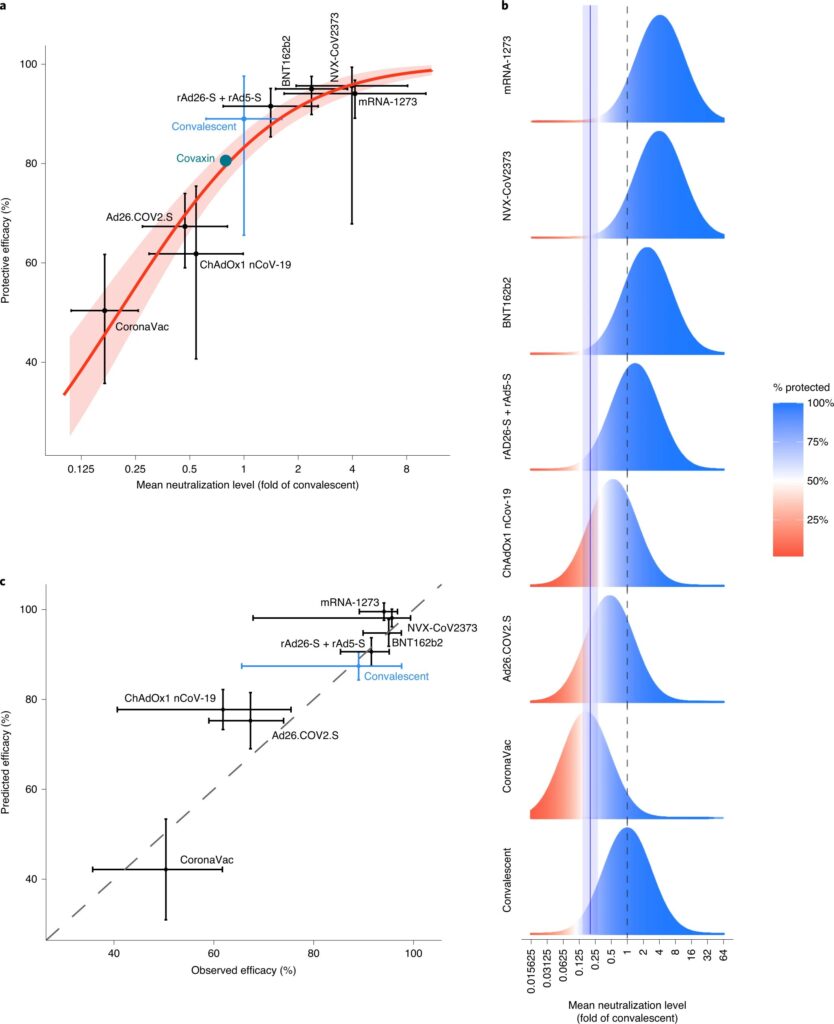
What Level of Neutralizing Antibodies Protects Against COVID-19
Both vaccination and natural infection with SARS-CoV-2 reduce the risk of reinfection and severe COVID-19. Patients who have developed antibodies to coronavirus in response to natural infection have 89% protection against reinfection. The effectiveness of the vaccine is from 50 to 95%.
Since immune responses weaken over time and defend less well against new strains of SARS-CoV-2, it is unknown how much coronavirus immunity lasts. One of the indicators by which one can judge how much a person is protected from SARS-CoV-2 infection is the level of neutralizing antibodies.
Australian scientists investigated how antibody levels after vaccination affect protection against SARS-CoV-2 infection. Scientists have compiled data on neutralizing antibody titers from seven phase 1 and 2 vaccines trials and studies involving patients recovering from COVID-19.
All vaccine trials used different methods of measuring neutralization and other criteria for recovery. Therefore, the scientists proposed normalizing the neutralizing antibody titers to the mean convalescent titer used in the same survey for each study.
The researchers then compared this normalized neutralizing antibody level in each study with the corresponding protective efficacy reported in seven phase 3 vaccine trials.
Research results:
- If the titer of neutralizing antibodies after vaccination is at least 20% of the average titer of convalescents – such a person is half as likely to be infected as an unvaccinated person. A 50% protective neutralization level, equivalent to 20% of the mean titer in convalescent subjects, corresponds to an in vitro measured neutralization titer of 1:10 to 1:30 in most of the assays described. It is estimated to be about 54 IU / ml.
- The level of neutralizing antibodies for 50% protection against severe infection is 3% of the average recovering level. This figure is well below the 20% level required to protect against the symptomatic disease of any severity.
- The titer of neutralizing antibodies is halved 108 days after vaccination. A new immunization may be required after a year. However, the level of neutralizing antibodies to protect against severe infection is 6 times lower than that needed to protect against the symptomatic disease of any severity. Therefore, protection against severe illness can be significantly more robust given the effect of T cells and memory B cells.
- A lower titer of neutralizing antibodies against new strains has a more substantial effect on vaccines for which the protective efficacy against wild-type virus is more deficient.
Relationship between neutralizing antibody levels and protection against SARS-CoV-2 infection (Figure b):
Simulating the protective neutralization level. Data for each study includes the distribution of the measured in vitro neutralization titer against SARS-CoV-2 in vaccinated or recovering subjects.
The dotted line is the mean titer in convalescent subjects.
The bell curve is the level of protective effectiveness. Effectiveness is illustrated by the proportions of the “protected” (blue) and “receptive” (red) bell curves.
The solid blue line is the optimal level of protective neutralization of 50% (half the chance of getting infected compared to an unvaccinated person).
Source: https://www.nature.com/articles/s41591-021-01377-8
Details of the study – in the article “What level of neutralizing antibodies protects against COVID-19.” The study was published in Nature Medicine.
How Many Antibodies Persist After Infection With Coronavirus
Neutralizing antibody titers correlate with protection against COVID-19 infection. Neutralizing antibody levels decrease over time after SARS-CoV-2 infection and vaccination.
In the first 3 months after infection, the level of antibodies decreases rapidly, and then the decline slows down. The first phase of antibody production is controlled by short-lived plasma cells, but longer-term antibody responses support antigen-specific long-lived plasma cells in the bone marrow.
Chinese scientists have estimated how long protection against recurrent COVID-19 lasts during a period when antibody levels decline slowly. Scientists monitored antibody levels in 124 people for 386 days from the onset of COVID-19 symptoms.
Facts about lowering antibody levels and protecting against re-COVID-19:
- The rate of decrease in the level of antibodies in children is much higher than in adults.
- Lighter COVID-19 is associated with a faster decline in neutralizing antibodies.
- Natural infection with SARS-CoV-2 protects 80.5% against reinfection, but for people over 65 years of age, the level of protection drops to 47.1%.
To estimate how much protection against reinfection is maintained, Chinese scientists used data that 20% of the average titer of antibodies recovering from natural SARS-CoV-2 halved the risk of infection.
Using this approach, the scientists calculated that 50% of patients recovering from symptomatic SARS-CoV-2 would be protected from reinfection by:
- 701 days (1.9 years) – based on antibody titers neutralizing 90% of viral particles;
- 990 days (2.7 years) – based on antibody titers that neutralize 50% of viral particles.
The neutralizing antibodies protect from reinfection with parental SARS-CoV-2 strains for about two years (700 days) after symptomatic COVID-19 infection. However, antibodies are less effective at neutralizing new strains of SARS-CoV-2, which may reduce, but not abolish, protection.
The duration of protection due to natural symptomatic infection cannot be directly extrapolated to vaccination results. The reasons are different neutralization titers and different rates of decline in antibody levels after infection and vaccination.
Details of the study – in the article “What level of neutralizing antibodies protects against COVID-19.” The research is published in The Lancet.
Could A Person with Antibodies to SARS-Cov-2 Be Infectious
Infection cannot be determined by antibody titers alone. Those who have been ill can shed the virus for a long time: a scientifically confirmed case when a person has shed the virus for 70 days.
Immunocompromised patients with COVID-19 can remain infectious for months and not develop antibodies.
Infectiousness reflects the result of the PCR test. The Ct value (cycle threshold) in PCR correlates with viral load. Low Ct values mean high viral load and may indicate a person is infectious:
- Ct <29 are strong positive reactions indicating an abundance of target nucleic acid in the sample.
- Ct 30-37 – Positive reactions indicate a moderate amount of target nucleic acid.
- Ct 38-40 – Weak reactions indicate a minimal amount of target nucleic acid. A high Ct level can show both a state of infection and environmental pollution.
Ct values can vary significantly between different tests and laboratories. In one study, samples with Ct values above 35 were positive for the virus in only 8% of cases. Since the Ct values were less than 35 in some reinfection cases, the virus could lurk in the nasal cavity and be infectious.
Coronavirus and Vaccination During Pregnancy: Are Antibodies Passed onto The Baby
The mother’s immune system plays a critical role during pregnancy, as the protection of the newborn from pathogens depends on the transfer of maternal IgG antibodies across the placenta. The coronavirus can interfere with the transmission of protective antibodies across the placenta.
Israeli scientists have assessed how efficiently the maternal body produces antibodies to coronavirus and transmits them through the placenta after vaccination and during natural infection with SARS-CoV-2 during pregnancy.
Antibodies in mother and fetus:
- Infection with SARS-CoV-2 in mid-pregnancy creates a sustained antibody response from the mother and the fetus.
- With natural infection with SARS-CoV-2, IgM antibodies in newborns indicate that the coronavirus has affected the fetus.
- When vaccinated, the response of fetal IgM antibodies to vaccine antigens is negligible. Vaccine antigens do not affect the fetus.
- Maternal IgG antibodies produced in response to vaccination are transmitted through the placenta to the fetus. It provides a protective titer of antibodies to SARS-CoV-2 in the bloodstream of newborns as early as 2 weeks after the first dose of the vaccine. The COVID-19 vaccine generates a steady level of maternal and fetal antibodies during pregnancy.
The study details are placed in the article “Vaccination of Pregnant Women: Newborn Receives Protective Maternal Antibodies to Coronavirus.” The study was published in The Journal of Clinical Investigation.
Antibodies Are Transmitted Through Breastfeeding
Both after COVID-19 and after vaccination, antibodies to coronavirus are found in breast milk. COVID-19 vaccines do not contain live viruses and therefore cannot cause infection. Vaccines do not pass into breast milk, but antibodies do.
After the second dose of the vaccine, the level of IgG antibodies to SARS-CoV-2, but not IgA, increases in maternal blood and breast milk, antibodies generated after vaccination neutralize SARS-CoV-2.
The antibodies in breast milk do not enter the baby’s bloodstream but cover their mouth instead, throat, and intestines before being wholly digested. However, until breastfeeding is stopped, these antibodies protect COVID-19. Therefore, the World Health Organization recommends that mothers continue to breastfeed after vaccination.
Details of the study are placed in the article “Vaccination against coronavirus during breastfeeding.” The research is published in Nature.
Neutralizing Antibodies in Children and Adults: Age and Disease Relation
The clinical manifestations of COVID-19 vary by age. Respiratory symptoms develop in adults. In their most severe form, they develop into acute respiratory distress syndrome (ARDS). Children often have no respiratory symptoms but can develop life-threatening multisystem inflammatory syndrome (MIS).
The neutralizing activity of antibodies to SARS-CoV-2 depends on the amount of S-IgG antibodies. In adult patients, the neutralizing activity of antibodies to SARS-CoV-2 does not depend on gender and age but depends on the severity of the disease. The more severe COVID-19, the higher the neutralizing activity of antibodies. Except for the critically ill adults with the ARDS group, neutralizing activity depends on time after symptom onset. The most increased neutralizing activity of antibodies is in adult patients with ARDS.
The antibody profile after SARS-CoV-2 infection is different in children and adults. In adults who have had coronavirus, antibodies to the viral spike protein (S, anti-spike antibodies) are produced: IgG, IgM, and IgA, and antibodies to viral RNA (N, antibodies to the nucleocapsid) IgG.
Regardless of the presence of MIS:
- Children develop anti-spike (S) IgG antibodies but no antibodies to viral RNA (N).
- In children, the neutralizing activity of antibodies is reduced in comparison with adults. Therefore the protective ability of antibodies in children is lower. In children without DIMS, the neutralizing activity of antibodies decreases with increasing age. This decrease corresponds to the fact that the number of IgG S antibodies in children drops by adolescence.
These differences are essential for developing testing strategies and protecting the population based on age.
Antibody Production Dynamics Depending on COVID-19 Severity
An international team of scientists from China, Saudi Arabia, and the United States in July 2020 investigated the dynamics of antibody production and viral load depending on the severity of COVID-19.
The study involved two groups of patients with a positive PCR test for COVID-19:
- The first group of 12 people, patients with a severe form of COVID-19. These people needed mechanical ventilation and were in intensive care units. All of them had severe pneumonia.
- The second group of 11 people, patients with a mild form of COVID-19. These people did not require intensive care. Their clinical presentation included fever, cough, malaise, headache, and mild pneumonia.
General dynamics of the production of IgM and IgG antibodies in COVID-19:
- IgM antibodies in patients with severe COVID-19 increased within 1–2 weeks of study initiation. After 4 weeks, antibodies gradually decreased.
- Patients with mild COVID-19 had significantly lower IgM antibodies. The majority of patients in this group (8/11) did not develop a noticeable amount of antibodies of this type during the entire period of the disease. Scientists emphasize that IgM diagnostics for patients with mild infection is not practical.
- IgG antibodies appeared after 10-15 days. Most of the patients had high levels of these antibodies, which persisted for at least one and a half months.
The dynamics of the production of IgM and IgG antibodies in different tissues:
- In seriously ill patients, IgM to SARS-CoV-2 was found in urine (3/10) and sputum (4/10). IgG antibodies were more often present in this group of patients: in urine (7/10) and sputum (7/10). In one severe case, IgM and IgG antibodies were detected in the bronchoalveolar fluid and pleural effusion. Takeaway: Severe coronavirus infection can damage various tissues, such as the respiratory tract and kidneys.
- In the group of patients with a mild form of the disease, antibodies to SARS-CoV-2 were not detected in sputum, bronchoalveolar fluid, pleural effusion, urine samples.
- In both groups, there was no antibody in the fecal samples.
Scientists suggest that the appearance of IgG to SARS-CoV-2 in urine and sputum may be a potential marker for determining the severity of the disease.
Which SARS-Cov-2 Proteins and How Far Do Antibodies React After The Onset of Symptoms
Scientists have compared the antigenicity of various SARS-CoV-2 proteins:
- S is the spike protein of the coronavirus;
- S1 – a fragment of the spike protein S, which is responsible for binding to the cell;
- RBD is an S1 fragment that directly binds to the cellular receptor ACE2.
- S2 – a fragment of the spike protein S, which is responsible for penetration into the cell;
- N – protein coat of the viral genome.
Research results:
- All proteins: S, S1, S2, RBD, N – were recognized by antibodies in the blood plasma. The peak of recognition was reached 3-4 weeks after the onset of the disease.
- Recognition of proteins S and S2 reached a maximum after 7-14 days. Recognition of proteins S1, RBD, N reached a maximum of 3 weeks after the onset of the disease.
- There were no apparent differences in IgG response against viral proteins between critically ill and mild patients.
Can You Get COVID-19 Again With Antibodies?
Reinfection with COVID-19 is possible even with a high titer of neutralizing antibodies. The earliest documented case of reinfection is 48 days later. In both cases of infection, the strains of the coronavirus were different. Reinfection with SARS-CoV-2 led to more severe illness than the first infection – oxygen support and hospitalization were required.
Are reinfections due to a weak antibody response after the first infection? Inadequate antibody responses are associated with suppressed immunity. However, an American study found that of the six reported cases of reinfection with coronavirus infection, none of the patients was found to be immunodeficient. After reinfection with coronavirus, only two people had serological data on the first infection, and at the time of reinfection, one person already had IgM antibodies against SARS-CoV-2.
Due to many serological testing platforms, it is impossible to compare one test result to another. For example, the presence of N antibodies indicates a previous infection with SARS-CoV-2 but does not guarantee that S antibodies are present that can block the infection. In addition, antibody levels are highly dependent on the time after infection.
Does immunity protect against reinfection? Not always. In some patients, the course of the disease with reinfection is more severe than with the first. It is important to remember that reinfection is usually due to symptoms, so unreported asymptomatic cases are highly likely. It means that it is unknown how often reinfection occurs among people who have recovered from the first infection. Instances of asymptomatic reinfection can only be detected during a routine examination or, for example, during testing at the airport.
Reinfection with coronavirus infection suggests that immunity acquired through natural infection cannot be relied upon to ensure herd immunity – vaccination is necessary. All people, whether previously diagnosed with COVID-19 or vaccinated, must take precautions to avoid contracting SARS-CoV-2.
Vaccinated COVID-19-Recovered Patients Have the Most Substantial Immunity to Coronavirus
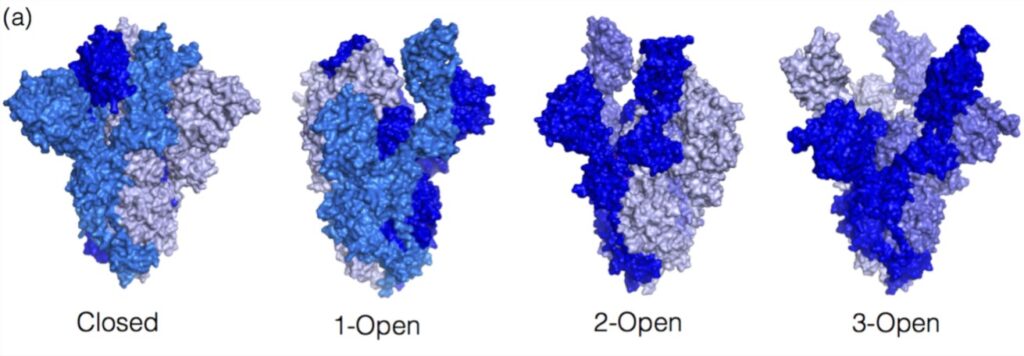
Only antibodies to the coronavirus spike protein (S-antibodies) have neutralizing activity. The spike protein changes in natural infection as the virus fuses with the cell, affecting the antibodies’ neutralizing activity.
The figure below shows the closed and open formations of the S protein. These forms differ in the position of the three RBD fragments of the spike protein: standing or lying. Before binding to ACE2 and entering cells, the S-protein remains in a closed-form – RBD fragments are supine. The open form of the spike protein is characterized by the standing position of the RBD, which allows the coronavirus to bind to the ACE2 receptor and enter the cell. Therefore, depending on whether the SARS-CoV-2 spike protein entered the body through ACE2 or with the help of a vaccine, antibodies to the S-protein will differ.
Image source: https://www.mdpi.com/1422-0067/23/4/2078/htm
Belgian scientists have found that a natural infection and a vaccine cause neu tralizing antibodies of different classes. After COVID-19, antibodies are produced that can bind to both closed and open formations of the spike protein. And after vaccination, antibodies are produced that can only bind to the open formation. Therefore, people who have had COVID-19 before or after immunization have the most robust and most stable immunity to coronavirus.
Neutralizing antibodies that recognize the S-protein in the closed state play a vital role in forming the immune response and protecting against infection. Antibodies to the closed formation of the S-protein block its transition to the open state and resist fusion with the cell. Therefore, the S-protein in the closed state can become the basis of a new generation of coronavirus vaccines.
Details of the study are published in the article “Post-Vaccination and Natural COVID-19 Antibodies: Which Protect Better?” The study was published in the International Journal of Molecular Sciences.
IgM Antibodies Protect Against Delta and Omicron Strains
Convalescent plasma or genetically engineered monoclonal antibodies are used to treat severe COVID-19. Convalescent plasma contains only 5% IgM antibodies, but IgM has a powerful neutralizing ability against coronavirus. This ability can be explained by the fact that IgM has 10 attachment points to viral particles or infected cells. However, in clinical practice, IgG monoclonal antibodies are used, which have only 2 attachment points but bind more strongly to foreign proteins.
New coronavirus strains contain mutations in the spike protein’s receptor-binding domain (RBD). Therefore, the effectiveness of monoclonal antibodies is reduced. However, multiple attachment points allow IgM antibodies to bind to SARS-CoV-2 viral particles with the same strength as IgG antibodies, which have a higher affinity for coronavirus RBDs.
American scientists compared the neutralizing activity of SARS-CoV-2-specific IgM and IgG and assessed how effectively these antibodies neutralize various coronavirus strains. Scientists have found that IgM retains a neutralizing ability to new strains of coronavirus, including Delta and Omicron, while the protective ability of IgG may be insufficient.
Research Results
- IgM enhances the effectiveness of neutralizing IgG antibodies. Expression of IgM required a 3-23 times lower concentration of IgG necessary to suppress the coronavirus. The neutralizing activity of serum containing IgM and IgG increased by 20–150 times.
- IgM neutralize coronavirus. The IgM antibody concentration is 66 times lower than IgG to suppress the coronavirus.
- IgM retains neutralizing activity against Delta and Omicron strains. Compared to IgG alone, the neutralizing activity of serum containing IgM and IgG increased by 4.8 and 6.4 times for the Delta and Omicron strains, respectively.
- IgM protects airway cells from coronavirus. IgG antibodies do not penetrate well into the lungs and respiratory epithelium, the target of SARS-CoV-2 infection. On the contrary, IgM can penetrate the mucosa. In addition, IgM activates protective blood proteins that directly target the pathogen and enhance the immune response.
- IgM can be administered intranasally and systemically to protect the respiratory epithelium.
The study is detailed in the article “IgM Antibodies Protect Against Delta and Omicron Strains.” The study was published in the Journal of Experimental Medicine.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary:
References
- Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City
- Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum
- Immune responses and immunity to SARS-CoV-2
- Reinfection with SARS-CoV-2: considerations for public health response
- What reinfections mean for COVID-19
- Kinetics of viral load and antibody response in relation to COVID-19 severity
- Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine
- COVID vaccines and breastfeeding: what the data say
- Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection
- Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection
- Analysis of the Neutralizing Activity of Antibodies Targeting Open or Closed SARS-CoV-2 Spike Protein Conformations
- IgM antibodies derived from memory B cells are potent cross-variant neutralizers of SARS-CoV-2