Convalescent plasma or genetically engineered monoclonal antibodies are used to treat severe COVID-19. These treatments provide passive immunity to the coronavirus.
Studies of the plasma of convalescents have shown that plasma contains only 5% IgM antibodies, but IgMs have a powerful neutralizing ability against coronavirus. This ability can be explained by IgMs having 10 attachment points to viral particles or infected cells. However, in clinical practice, IgG monoclonal antibodies are used, which have only 2 attachment points but bind more firmly to foreign proteins.
New coronavirus strains contain mutations in the spike protein’s receptor-binding domain (RBD). Therefore, the effectiveness of monoclonal antibodies decreased. There are two ways to solve this problem:
- develop cross-variant neutralizing monoclonal antibodies;
- modify existing monoclonal antibodies, so that coronavirus mutations affect them less.
In a previous study, scientists found that natural IgM or artificially created IgG with 6 attachment points enhances the protective function of malaria monoclonal antibodies. This effect is because antibodies bind more strongly to the surface containing foreign proteins due to the multitude of attachment points.
Therefore, an increase in attachment points can compensate for a decrease in the affinity of monoclonal antibodies to mutated RBD coronavirus. Another study confirmed this hypothesis: artificially created antibodies with many attachment points neutralized the mutant human immunodeficiency virus. In addition, the coronavirus spike proteins are distributed with such a density that IgM can bind several spike proteins of one or two neighboring viral particles.
IgM antibodies and IgM-secreting B cells are involved in an early response to infection and provide unstable immunity until IgG antibodies appear, which bind more firmly to foreign proteins. The later response to infection involves plasmablasts secreting antibodies, memory B cells, and plasma cells that emerge from germ centers. When antigen-specific memory B cells are reactivated, they produce antibodies that can quickly suppress the pathogen of infection.
After coronavirus infection and vaccination, SARS-CoV-2 are formed-specific memory B cells that produce IgG antibodies and memory B cells that have IgM to a lesser extent.
American scientists compared the neutralizing activity of SARS-CoV-2-specific IgM and IgG and assessed how effectively these antibodies neutralize various coronavirus strains. In addition, the scientists tested whether the memory B cells contained in the plasma of convalescents produce IgM antibodies capable of binding and neutralizing the coronavirus. Scientists have found that IgM can play an essential role in protecting against new coronavirus strains, including Delta and Omicron, while the protective ability of IgG and memory B cells that produce IgG may be insufficient.
Research Results
IgMs increase the effectiveness of neutralizing IgG antibodies produced by memory B cells
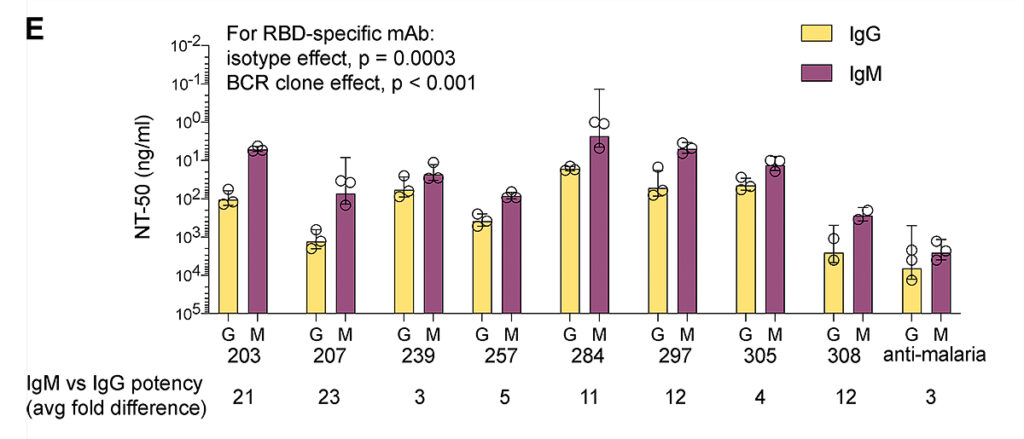
IgM expression required 3-23 times less IgG concentration to suppress coronavirus by 50%. The neutralizing activity of the serum containing IgM and IgG increased 20-150 times:
Image source: https://rupress.org/jem/article/219/9/e20220849/213384/IgM-antibodies-derived-from-memory-B-cells-are
The liquid part of the B cells bound the RBD of the coronavirus if it contained IgM. Moreover, the IgM-producing B cells’ liquid part prevented the RBD coronavirus from binding to the ACE2 receptor.
The level of memory B cells that produce IgM (IgM+ MBC) decreases for several months after COVID-19 in unvaccinated individuals. However, long-lived IgM+ MBC can persist for a year after infection with coronavirus.
IgMs produced by IgM+ MBC neutralize COVID-19
A comparison of IgG and IgM showed that the concentration of antibodies required to suppress coronavirus by 50% was 66 times lower for IgM than for IgG. Thus, 400 times fewer IgM molecules were required than IgG.
These results confirm that the multiple attachment points of IgM antibodies allow IgM antibodies to bind to SARS-CoV-2 viral particles with the same strength as IgG antibodies, which have a greater affinity for RBD coronavirus.
Monoclonal IgM antibodies retain neutralizing activity against Delta and Omicron strains
The Omicron strain was intensely mutated, so the existing IgG monoclonal antibodies either did not neutralize or only partially neutralized it. However, monoclonal IgM antibodies retained neutralizing activity at concentrations <2 mcg/ml. For 50% neutralization of Delta and Omicron coronavirus strains, IgG concentrations of 111 and 147 ng/ml, respectively, are required. IgM improved the neutralization efficiency to 23 ng/ml against both strains.
IgM protects respiratory tract cells from COVID-19
IgG antibodies do not penetrate well into the lungs and respiratory epithelium – the main target of SARS-CoV-2 infection. On the contrary, IgM can penetrate the mucous membrane.
IgMs are essential for long-term protection against respiratory pathogens. One of the problems with the therapeutic use of IgM is the short half-life in serum compared to IgG. However, a recent study showed that IgM delivered to the nasal passages protected mice from the coronavirus.
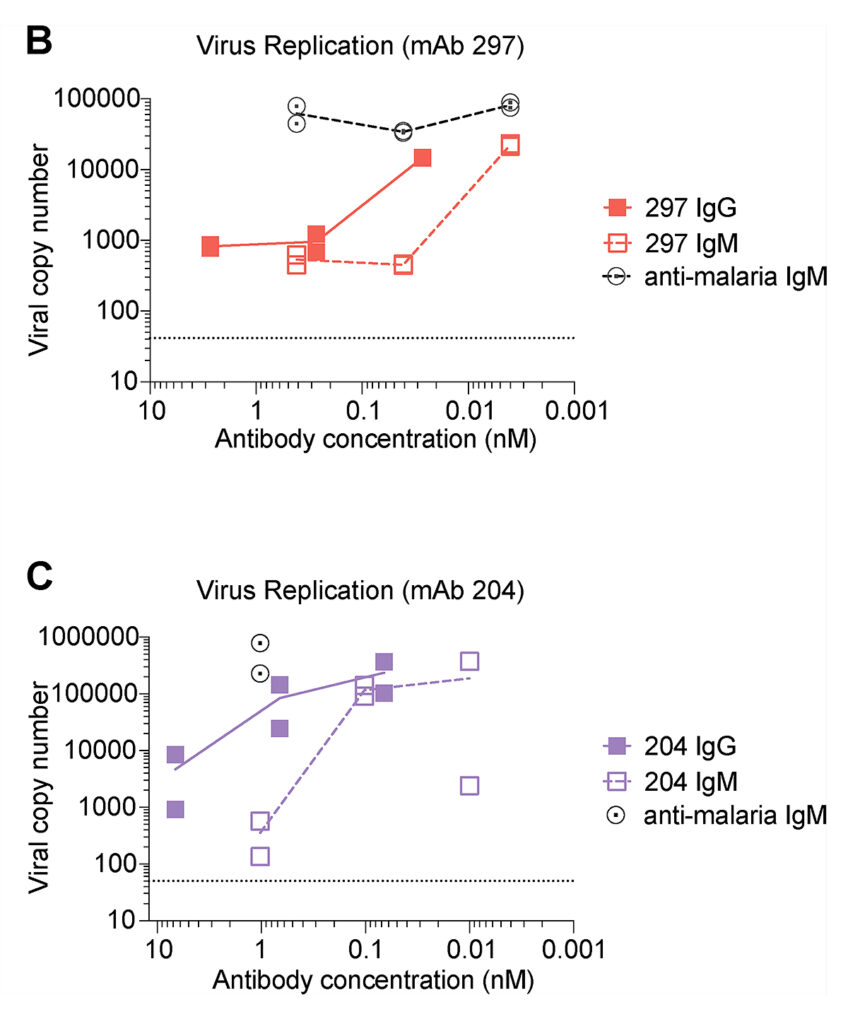
On the culture of respiratory epithelial cells, American scientists showed that both IgG and IgM reduced the number of viral copies in a dose-dependent manner, and a lower dose of IgM was required.
Image source: https://rupress.org/jem/article/219/9/e20220849/213384/IgM-antibodies-derived-from-memory-B-cells-are
Scientists have also studied the protective ability of IgA antibodies. In an experiment on IgA cells, coronavirus was neutralized more effectively than IgG but less effectively than IgM. IgA is more efficiently transported to the mucous membrane of the respiratory tract, while IgM is maintained at higher concentrations in the blood, in addition to access to the mucous membrane. Unlike IgA, IgMs activate protective blood proteins that directly target the pathogen and enhance the immune response. Therefore, the delivery of IgM to the mucous membrane of the respiratory tract can achieve both therapeutic effects. Furthermore, systemic delivery can increase IgM stability on the mucous membrane’s surface.
Conclusion
Multiple attachment points and increased binding resistance to new strains of SARS-CoV-2 make IgM antibodies powerful neutralizers of a wide range of coronavirus strains. IgM monoclonal antibodies may be beneficial for the treatment of patients with COVID-19.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary:
Reference
IgM antibodies derived from memory B cells are potent cross-variant neutralizers of SARS-CoV-2