Although the coronavirus is primarily a respiratory infection, it causes extrapulmonary complications in severe cases. Coronavirus can lead to cardiovascular damage. Some patients with COVID-19 have elevated troponin levels, a marker of heart damage, and impaired cardiac function. Cardiovascular complications associated with COVID-19 include myocarditis, acute myocardial infarction, heart failure, and arrhythmias.
Other infections may affect the course of the coronavirus. Several studies have shown that coinfection with the influenza A virus (IAV) worsens the outcome of coronavirus.
In an experiment in mice, the influenza A virus upregulated the expression of ACE2, the SARS-CoV-2 receptor, in the lungs, promoting infection.
ACE2 is expressed in many tissues throughout the body, which allows the coronavirus to infect cells in the lungs and the heart. In addition, the influenza A virus can infect heart tissue and cause cardiac fibrosis and electrical dysfunction.
ACE2 Role During Infection
The ACE2 enzyme is required to convert angiotensin II (Ang II) to angiotensin 1-7. Hormones Ang II and Ang 1-7 have the opposite effect:
- Ang II promotes vasoconstriction, inflammation, cell death, fibrosis, hypertrophy and tissue remodeling.
- Ang 1-7 promotes the expansion of blood vessels and has an antioxidant and anti-inflammatory effect.
For the ACE2 enzyme to convert Ang II to Ang 1-7, it must combine with a carbohydrate – undergo glycosylation. At the same time, the glycosylation type determines whether ACE2 can bind to the coronavirus spike protein.
American scientists have shown that the influenza A virus changes the expression and glycosylation of ACE2 and investigates how the flu affects the subsequent infection of heart cells with coronavirus.
Influenza Virus Upregulates ACE2 Expression but Does Not Affect TMPRSS2
To determine how the influenza A virus affects the expression of ACE2, the scientists used 4 types of cells:
- epithelial cells of the lungs;
- lung fibroblasts – connective tissue cells;
- cardiac fibroblasts;
- cardiomyocytes – muscle cells of the heart.
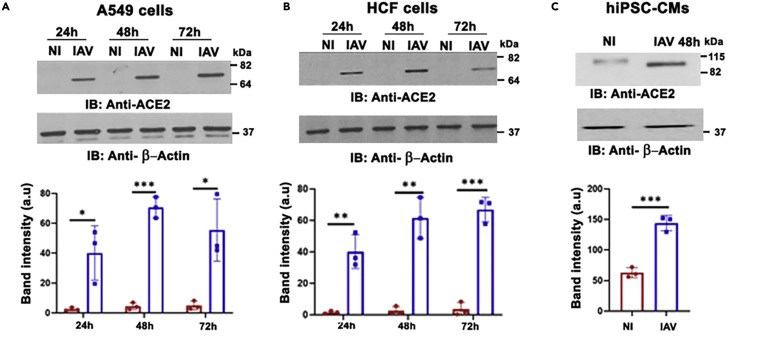
In uninfected lung epithelial cells and pulmonary and cardiac fibroblasts, ACE2 was weakly expressed. However, the influenza A virus significantly upregulated ACE2 expression in these cells.
In uninfected cardiomyocytes, ACE2 was expressed at a detectable level. Expression was further increased upon infection with the influenza A virus.
Figure 1. Influenza A virus upregulates ACE2 expression in heart cells
(A) Lung epithelial cells – A549, (B) cardiac fibroblasts – HCF, (C) cardiomyocytes – hiPSC-CMs.
Image source: https://www.cell.com/iscience/fulltext/S2589-0042(22)01974-5
The lung tissue, the primary site of SARS-CoV-2 infection, is composed primarily of epithelial cells but also contains large numbers of fibroblasts and macrophages. Influenza A virus upregulates ACE2 expression in bronchial epithelium, lung fibroblasts, and macrophages.
The data from the cell experiments were confirmed when the scientists examined lung samples from patients who had died of influenza A and B viruses. Unlike lung tissue taken from patients who had died of other causes, the lungs of patients who had died of influenza overexpressed ACE2.
However, when scientists examined cardiomyocytes, it turned out that these cells were more resistant to the influenza virus. Influenza A virus caused the death of cardiac fibroblasts but did not affect the survival of cardiomyocytes.
Next, the scientists investigated the TMPRSS2 enzyme, which is also essential for entering the coronavirus into the cell. Influenza A virus did not affect the expression of TMPRSS2 in any of the cell types studied.
Interferon Regulates ACE2 Expression in The Heart
In addition to experiments on cells, scientists experimented on mice, infecting them with the influenza virus. Influenza increased ACE2 expression in the lungs and heart of mice. Moreover, intranasal influenza infection causes cardiac fibrosis and electrical dysfunction.
ACE2 is a product of interferon-stimulated genes. The influenza virus elicited an interferon-beta (IFN) response in heart tissue. IFN-beta levels correlated with the severity of the infection, meaning that the influenza virus-driven interferon response plays a vital role in upregulating ACE2 expression in the heart.
Influenza, which preceded coronavirus infection, increased the mortality of mice. Influenza increased ACE2 expression in the lungs and heart, promoting entry and replication of SARS-CoV-2.
Influenza Virus Alters ACE2 Glycosylation, Affecting Susceptibility to Coronavirus
Glycosylation is required for ACE2 enzymatic activity, i.e., to convert Ang II to Ang 1-7. In addition, glycosylation affects the binding of ACE2 to the coronavirus spike protein.
ACE2 in the lungs. In influenza-infected epithelial cells of the lungs and heart fibroblasts, ACE2 is not glycosylated and does not have enzymatic activity. However, non-glycosylated ACE2 in these cells can bind to the coronavirus spike protein. An experiment on lung samples showed that the influenza virus enhances the binding of the coronavirus spike protein to the ACE2 receptor, facilitating the entry and replication of the coronavirus.
ACE2 in the heart. Glycosylated ACE2 was found in influenza-infected cardiomyocytes. The influenza virus enhances ACE2 enzymatic activity in these cells. If in cardiac fibroblasts, the influenza virus enhances the penetration and replication of the coronavirus, then in cardiomyocytes, on the contrary, the influenza virus suppresses the replication of the coronavirus.
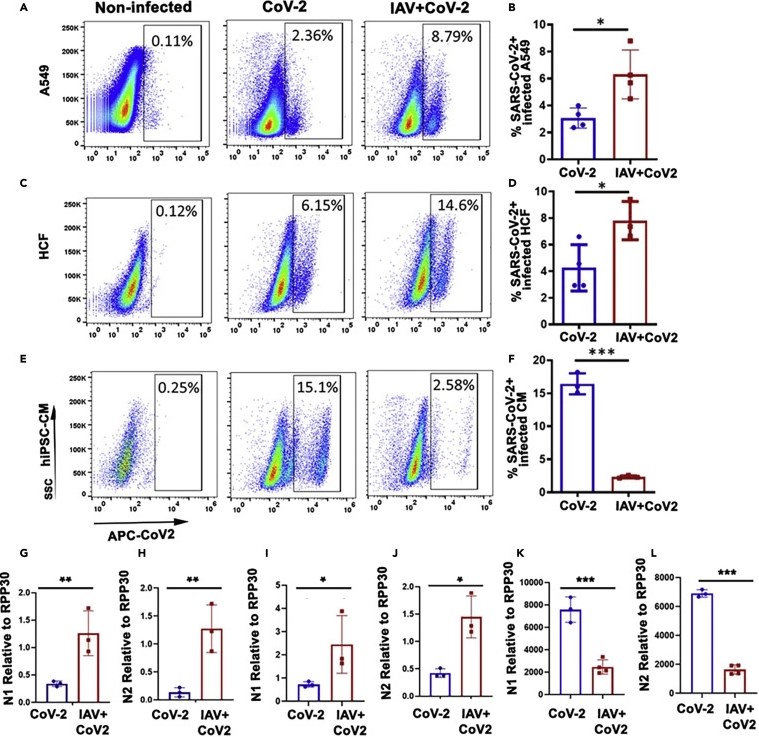
Figure 2. Influenza A virus enhances the infectivity of SARS-CoV-2
A549, lung epithelial cells; HCF, cardiac fibroblasts; hiPSC-CMs, cardiomyocytes. Graphs (A-F) demonstrate increased SARS-CoV-2 infectivity in influenza-infected lung epithelial cells and cardiac fibroblasts but not in cardiomyocytes.
Graphs (G-L) show the relative expression of SARS-CoV-2 N protein mRNA in A549 (G, H), HCF (I, J) and hiPSC-CM (K, L) cells infected with SARS-CoV-2 or influenza alone and SARS-CoV-2.
Image source: https://www.cell.com/iscience/fulltext/S2589-0042(22)01974-5
Influenza Virus Increases The Level of Angiotensin II In Lung Epithelial Cells and Cardiac Fibroblasts
ACE2 converts Ang II to Ang 1-7, suppressing the pro-inflammatory response triggered by Ang II.
The influenza virus increases ACE2 expression in lung epithelial cells and cardiac fibroblasts but does not increase ACE2 enzymatic activity. Influenza-infected lung epithelial cells and cardiac fibroblasts produce Ang II, which is not converted to Ang 1-7.
On the contrary, in cardiomyocytes, the influenza virus increases the expression of ACE2 and its enzymatic activity. In cardiomyocytes, the influenza virus significantly reduces Ang II levels due to increased expression of glycosylated ACE2 in cardiomyocytes, which converts pro-inflammatory Ang II into immunosuppressive Ang 1-7, creating conditions for secondary coronavirus infection in an immunosuppressed environment.
Influenza Virus Enhances Nitric Oxide Production in Cardiomyocytes, Suppressing Coronavirus
The conversion of Ang II to Ang 1-7 leads to increased production of nitric oxide NO2. Pre-infection of cardiomyocytes with influenza virus increases NO2 levels due to a decrease in Ang II levels. However, influenza infection of lung epithelial cells and cardiac fibroblasts does not cause an increase in NO2 due to high levels of Ang II.
NO2 inhibits coronavirus replication in cardiomyocytes. However, cardiomyocytes are metabolically active, so NO2 can cause contractile dysfunction and death. The scientists disproved this possibility by showing that SNAP, a drug that boosts nitric oxide production, increased the beats per minute immediately after supplementation, but the beats returned to normal within 3 hours. In addition, the structure of the cells during this time did not change, and the cells did not die, demonstrating that NO2 is not toxic to cardiomyocytes.
SNAP treatment also increased NO2 production in influenza- and coronavirus-infected cardiac fibroblasts. NO2 contributed to the suppression of the coronavirus in these cells.
Output
The influenza virus upregulates the expression of the ACE2 coronavirus receptor and alters its glycosylation. The ability of ACE2 to participate in the conversion of pro-inflammatory Ang II to immunosuppressive Ang 1-7, as well as to bind to the coronavirus spike protein, depends on glycosylation.
Influenza-altered glycosylation differs between lung epithelial cells, cardiac fibroblasts, and cardiomyocytes and affects the outcome of coronavirus infection differently.
Influenza increases the infectivity of coronavirus in lung epithelial cells and cardiac fibroblasts. However, in cardiomyocytes, influenza pre-infection significantly reduces coronavirus replication without affecting SARS-CoV-2 entry.
Increased expression of ACE2 and its enzymatic activity in cardiomyocytes promote the conversion of Ang II to Ang 1-7, enhancing nitric oxide production. Nitric oxide inhibits coronavirus replication.
Coronavirus is sensitive to interferons. The influenza virus causes an interferon response. However, interferon-beta levels do not differ between lung epithelial cells, cardiac fibroblasts, and cardiomyocytes despite a significant decrease in SARS-CoV-2 replication and viral load in cardiomyocytes. The suppression of coronavirus replication in cardiomyocytes does not depend on the interferon response triggered by the influenza virus.
At the same time, the influenza virus enhances the production of nitric oxide in cardiomyocytes but not in the epithelial cells of the lungs and cardiac fibroblasts. Therefore, it can be concluded that the suppression of coronavirus replication in cardiomyocytes depends on the production of nitric oxide, which has an antimicrobial effect against various pathogens, including DNA and RNA viruses.
Pre-coronavirus influenza infection increases the severity of coronavirus in the lungs but protects cardiomyocytes from SARS-CoV-2. The flu shot may protect the heart by increasing the expression of glycosylated ACE2 and also protect the lungs by enhancing the interferon response, which reduces the severity of SARS-CoV2 in the lungs.
The flu shot can help cure a lung infection in coronavirus patients without worrying about heart dysfunction. Drugs that specifically increase glycosylation of the ACE2 protein and nitric oxide boosters may also be helpful in the treatment of lung infections caused by influenza and coronavirus.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary:
Reference
Influenza A virus modulates ACE2 expression and SARS-CoV-2 infectivity in human cardiomyocytes