People with weakened immune systems are at increased risk of severe coronavirus infection. In the United States, the proportion of these people is about 3% of the adult population. Vaccination of immunocompromised people is a problem because these people do not develop protective immunity levels after the vaccine is given.
Immunocompromised people include those who:
- Received active treatment for cancer of the internal organs or blood.
- Received an organ transplant and is taking medication to suppress the immune system.
- Received a stem cell transplant in the past 2 years and is taking medication to suppress the immune system.
- Suffers from moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome.
- Suffers from advanced or untreated HIV infection.
- Was treated with high doses of corticosteroids or other drugs that may suppress the immune response.
When infected with the SARS-CoV-2 coronavirus, immunocompromised patients have a higher risk of:
- Prolonged infections and virus shedding. This increases the likelihood of disease in relatives.
- Evolution of the virus. It is one of the reasons for the emergence of new strains of coronavirus.
- Low titers of antibodies in general and neutralizing antibodies in particular. This increases the likelihood of severe illness and re-infection.
An American study of the effectiveness of the COVID-19 vaccine found that 44% of vaccinated people who still contracted the coronavirus and were hospitalized are immunocompromised. An Israeli study showed similar results: 40% of those vaccinated but infected and hospitalized are people with weakened immunity.
How effective are coronavirus vaccines for immunocompromised people
Below are the results of studies on the effectiveness of mRNA vaccines.
Effectiveness in 7-27 days after the second dose of Pfizer-BioNTech vaccine :
- 71% among immunocompromised people versus the usual 90%. The rest have SARS-CoV-2 infection.
- 75% among people with weakened immunity versus 94%. The rest have COVID-19 with symptoms.
Effectiveness in 7 days after the second dose of Pfizer-BioNTech/Moderna vaccine:
- 80% of people with inflammatory bowel disease taking immunosuppressive drugs. The rest have SARS-CoV-2 infection.
- 25% of SARS-CoV-2 infections – after the first dose of mRNA vaccine.
Effectiveness in 14 days after the second dose of Pfizer-BioNTech/Moderna vaccine:
- 59% among immunocompromised people versus 91% without immunodeficiency. The rest have hospitalizations due to COVID-19.
Figure 1. Percentage of subjects with antibody response after vaccination with two doses of mRNA vaccine. Various immunodeficiency states data
Source: The US Centers for Disease Control and Prevention (CDC)
Immunocompromised people need a third dose of coronavirus vaccine
The US Centers for Disease Control and Prevention (CDC) recommends that people with moderate to severely immunocompromised immunity receive an additional dose of COVID-19 mRNA vaccine after the first two doses.
An additional dose of COVID-19 mRNA vaccine should be the same as in the original batch. The third dose should be given at least four weeks after the completion of the entire vaccination.
Comparison of the effectiveness of second and third doses of COVID-19 vaccine in immunocompromised and seropositive people
| 2nd dose of vaccine | 3rd dose of vaccine
Seronegative after 2nd dose |
|||||
| Patients population | Group size | Number of seronegative patients
N (%) |
Number of seropositive patients
N (%) |
Group size | Number of seronegative patients
N (%) |
Number of seropositive patients
N (%) |
| Organ transplantation | 99 | 59 (60) | 40 (40) | 59 | 33 (56) | 26 (44) |
| Organ transplantation * | 30 | 24 (80) | 6 (20) | 24 | 16 (67) | 8 (33) |
| Hemodialysis | 82 | 13 (16) | 69 (84) | 12 | 7 (58) | 5 (42) |
| Hemodialysis | 106 | 66 (62) | 40 (38) | 12 | 6 (50) | 6 (50) |
* Among those who did not have a detectable antibody response to the batch of the original mRNA vaccine, 33-50% developed an antibody response to the additional dose.
Figure 2. Effectiveness of three doses of COVID-19 vaccine among transplant recipients
Source: US Centers for Disease Control and Prevention (CDC)
No severe side effects were reported after the 3rd dose of the vaccine, and there were no episodes of acute rejection.
Third dose side effects
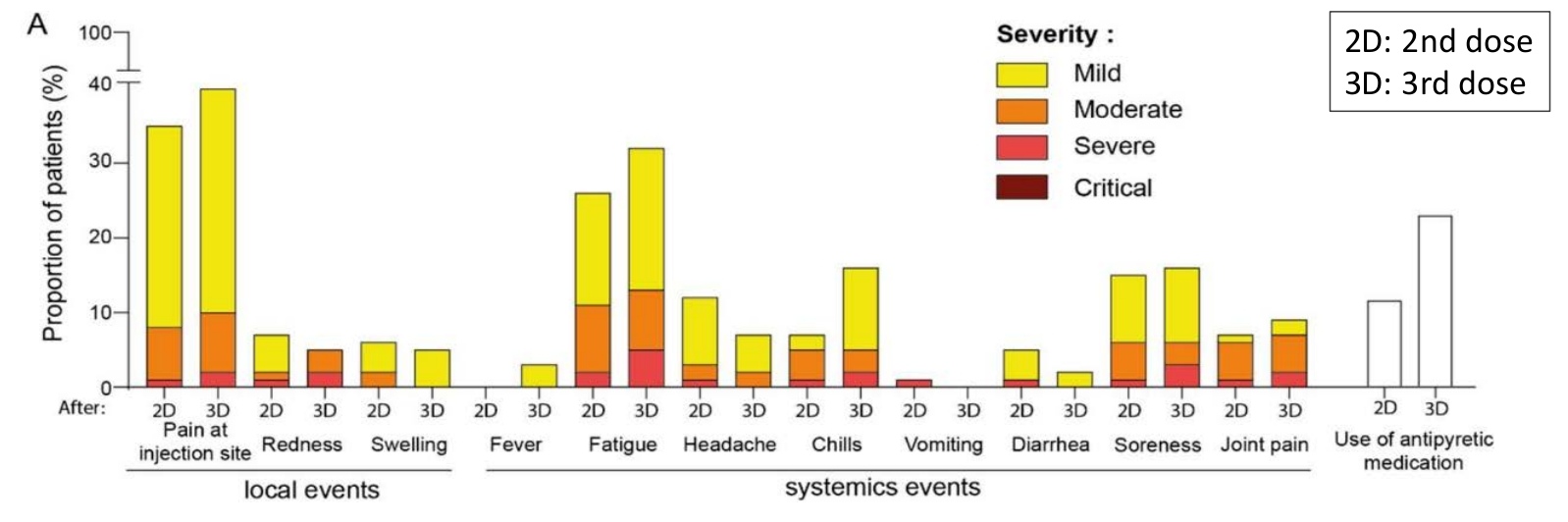
The sample included patients who had an optimal and suboptimal antibody response to a series of primary mRNA and chose the third dose. No patient developed acute side effects requiring hospitalization. The reported symptoms were consistent with previous doses and intensity and were primarily mild to moderate. The most common side effects of the third dose of the vaccine were fatigue and pain at the injection site.
Figure 3. Reactogenicity of the 3rd dose of mRNA vaccine in a cohort of hemodialysis patients
Source: US Centers for Disease Control and Prevention (CDC)
Output
Immunocompromised people respond poorly to two doses of the COVID-19 vaccine. The CDC recommends that people with moderate to severely weakened immunity receive an additional dose of the same vaccine to enhance protection. Although an extra dose of the vaccine can improve the security of immunocompromised people, even after being vaccinated, these people should follow preventive measures to protect themselves and those around them from COVID-19.
Sources
COVID-19 Vaccines for Moderately to Severely Immunocompromised People
Data and clinical considerations for additional doses in immunocompromised people