Between 3-10% of MS patients experience symptoms before the age of 18, predominantly in the form of relapsing-remitting MS (RRMS). RRMS is characterized by alternating periods of relapse and relative improvement. Children with RRMS often recover well after a relapse but typically accumulate disability within 15 years of onset, earlier than in adults.
Symptoms of MS can include:
- optic neuritis,
- sensory and motor impairments,
- cerebellar and brainstem dysfunction
50-70% of children experience multiple symptoms simultaneously.
The McDonald and Poser criteria, which include clinical and magnetic resonance imaging (MRI) data, are used for MS diagnosis.
Children with MS face unique challenges, including physical disability, cognitive impairment, fatigue, and psychological issues. They often experience relapses earlier than adults, leading to earlier disability. Fatigue can impact school attendance, and these children may exhibit reduced processing speed, attention, verbal working memory, abstract thinking, and the ability to coordinate visual perception with motor activity.
Given the faster progression of the disease in children, starting MS treatment early is crucial. Interferon beta-1b and glatiramer acetate are commonly used, demonstrating a favorable safety profile, reducing relapse frequency, and slowing disease progression.
Interferon Beta-1b for Pediatric MS Treatment
German scientists explored the effectiveness and safety of interferon beta-1b in treating children with relapsing-remitting multiple sclerosis (RRMS). In the study, 65 children aged 12 to 16 with RRMS received subcutaneous injections of interferon beta-1b every other day at a dosage of 250 µg. Participants were monitored for two years with clinic visits every six months, and 34 patients completed the study.
The evaluation of treatment effectiveness and safety was based on:
- Relapse frequency of MS
- Disability progression
- Changes in MRI
- Fatigue
- Psychological tests
Study results indicated that interferon beta-1b injections can effectively and safely treat MS in children.
Effectiveness
Most participants experienced a reduced frequency of MS relapses:
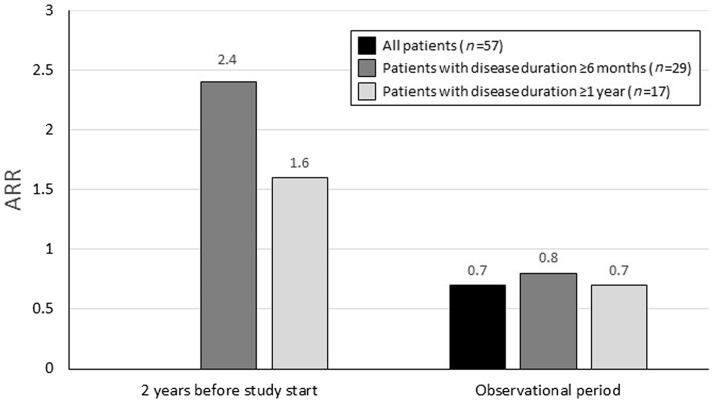
- For children with MS, duration over a year: 0.7 relapses per year during the study compared to 1.6 before the study.
- For children with MS, the duration is over six months: 0.8 relapses per year during the study compared to 2.4 before the study.
MRI studies showed a decreased number and volume of new demyelination foci in the brain.
Until the last observation, disability did not increase in 76.9% of participants.
Fig. 1. Annual relapse rate (ARR) before and during the study
Image source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5753955/
Safety and Side Effects:
- Overall, interferon beta-1b was well-tolerated.
- The most common side effects included injection site reactions, flu-like symptoms, headache, and elevated liver enzyme levels.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary: