Despite vaccination efforts, hepatitis B remains a serious global issue, especially for children who have not completed full vaccination. Hepatitis B often progresses to a chronic form in children. Therefore, hepatitis B treatment should commence as early as possible.
Interferon alpha (IFN-α) has been used to treat hepatitis B in children since the 1980s. Recent studies suggest that IFN-α treatment lasting over a year is safe and more effective than short-term therapy lasting 3-6 months. Prolonged treatment helps reduce viral activity and decreases the risk of cirrhosis and liver cancer.
Chinese scientists conducted a 13-year study to evaluate interferon-alpha treatment’s long-term effectiveness and safety for children with chronic hepatitis B. The study included children aged 1 to 17 with active chronic hepatitis B. With parental and child consent, the children were divided into two groups:
- 316 individuals received 72 weeks of interferon-alpha treatment (intramuscular IFN-α injections at a dosage of 3-5 MU/m2).
- 95 individuals comprised the control group.
The 72-week IFN-α therapy significantly improved the clearance of hepatitis B virus antigens, HBeAg and HBsAg, compared to the control group. In the IFN group, there was a higher likelihood of:
- Normalization of ALT levels in blood serum (55.70% vs 36.80%)
- Reduction of hepatitis B virus DNA to <2000 IU/mL (41.46% vs 27.37%)
- Negative HBeAg analysis for hepatitis B virus (39.87% vs 27.37%)
- Negative HBsAg analysis for hepatitis B virus (11.08% vs 3.16%)
Effectiveness of IFN-α for Hepatitis B Treatment in Children
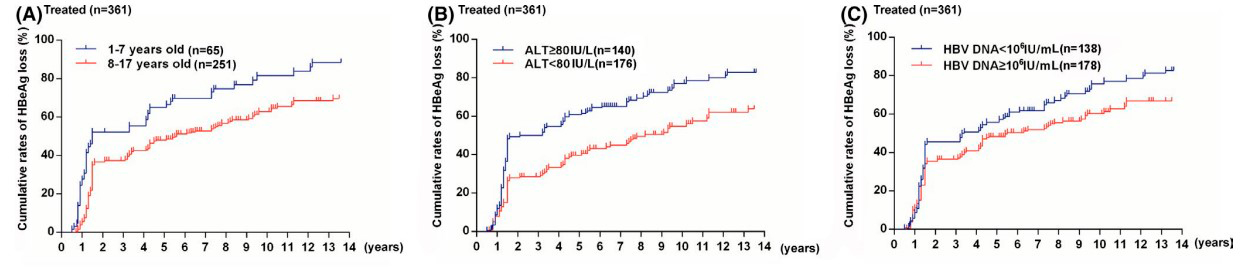
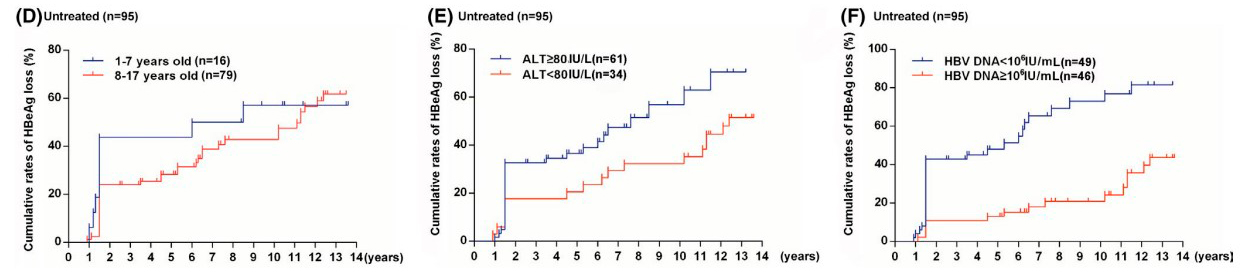
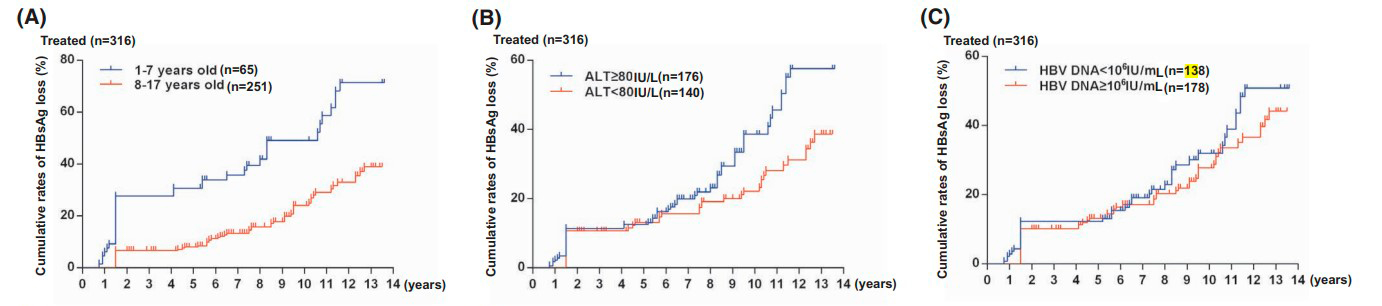
The effectiveness of interferon-alpha treatment was assessed at 5 and 13 years based on HBeAg and HBsAg clearance. Over 5 years, negative HBeAg and HBsAg analyses were more common in the IFN group, and this trend persisted over 13 years. Younger age, higher ALT levels, and lower hepatitis B virus DNA levels in children at the beginning of the study were associated with better HBeAg clearance results. Children aged 1-7 years in the IFN group had a higher likelihood of negative HBsAg analysis. Children who became HBeAg-negative by the 72nd week had a higher probability of negative HBsAg analysis. In the control group, the results were not age-dependent and were not affected by ALT or hepatitis B virus DNA levels.
Figure 1: HBeAg clearance rates in patients treated with interferon-based on:
- Initial age
- Initial ALT levels in blood serum
- Initial hepatitis B virus DNA levels in blood serum
Image source: https://onlinelibrary.wiley.com/doi/full/10.1111/jvh.13598
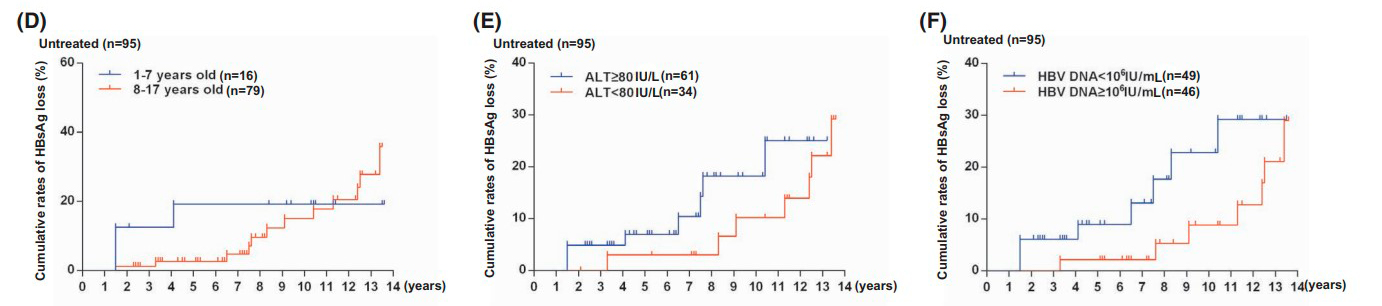
Figure 2: HBeAg clearance rates in patients in the control group based on:
- (D) Initial age
- (E) Initial ALT levels in blood serum
- (F) Initial hepatitis B virus DNA levels in blood serum
Image source: https://onlinelibrary.wiley.com/doi/full/10.1111/jvh.13598
Figure 3: HBsAg clearance rates in patients treated with interferon-based on:
- Initial age
- Initial ALT levels in blood serum
- Initial hepatitis B virus DNA levels in blood serum
Image source: https://onlinelibrary.wiley.com/doi/full/10.1111/jvh.13598
Figure 4: HBsAg clearance rates in patients in the control group based on:
- (D) Initial age
- (E) Initial ALT levels in blood serum
- (F) Initial hepatitis B virus DNA levels in blood serum
Image source: https://onlinelibrary.wiley.com/doi/full/10.1111/jvh.13598
Safety of IFN-α for Hepatitis B Treatment in Children
Interferon alpha treatment was well-tolerated. The most common side effects included:
- Fever (85.76% of children)
- Neutropenia (74.68%)
- Decreased appetite (45.89%)
- Fatigue (33.23%)
- Thyroid function abnormalities (28.16%)
- Hair loss (20.75%)
Other side effects, such as rash, joint pain, sleep disturbances, and febrile seizures, were rare, occurring in less than 10% of children. No child experienced an increase in ALT levels of more than 10 times the initial value. All side effects were resolved by the end of treatment, except for three cases of thyroid function abnormalities, which normalized during the one-year follow-up.
Although children’s weight and growth rate tended to decrease by the end of treatment compared to initial values, these measures recovered and exceeded the initial levels by the one-year follow-up. Children’s growth had no significant impact by the 72nd week of IFN-α treatment.
Conclusion
A 72-week interferon-alpha treatment was effective and well-tolerated by children with active chronic hepatitis B. Treatment significantly improved the clearance of HBeAg and HBsAg in the short and long term. Better outcomes were observed in children under 7 years old and those with higher ALT levels.
Side effects of treatment were mild to moderate and did not necessitate therapy discontinuation. Treatment did not affect the children’s growth.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary: