Why do you need a thymus?
The thymus or thymus gland is the primary lymphoid organ in which immune cells develop and mature, which then spread throughout the body. These cells destroy malignant neoplasms – cancer cells and fight a wide range of foreign antigens: viruses, bacteria, parasites, and fungi.
The thymus is fragile and sensitive to external influences. It constantly experiences cycles of degradation and regeneration. Thymus degeneration contributes to the following:
- attacks by alien organisms;
- damage caused by drugs, ionizing radiation, and toxins;
- hormonal changes.
After damage, the thymus is restored, but with age, the regeneration process deteriorates, and the gland degrades, which is manifested in a decrease in the size of the thymus and a deterioration in its architecture. As a result, the number and variety of immune cells maturing in it is reduced.
The most severe degradation and dysfunction of the thymus gland are manifested in the form of:
- relapses of oncological diseases;
- autoimmunity, when immune cells attack healthy organs;
- poor clinical outcome in infections that do not cause disease in a person with a healthy thymus.
This review lists factors that cause thymus degeneration and strategies to improve thymus regeneration.
Factors of damage and degradation
Acute Trauma
Glucocorticoids cause acute degradation of the thymus gland. Both drugs of this class, such as dexamethasone and glucocorticoids, produced by the body are destructive. For example, during stress or in violation of the mechanisms of regulation of adrenal function, an excessive amount of the hormone cortisol, a natural glucocorticoid, is produced.
As Hungarian scientists showed in 2002, glucocorticoids contribute to the programmed death of immature thymocytes (thymus cells). Italian scientists in 2003 confirmed this discovery by showing that the synthetic glucocorticoid dexamethasone stimulates the production of proteins in thymocytes that cause their programmed death. Moreover, Chinese scientists 2018 clarified that dexamethasone damages the cells of the thymus itself and changes its architecture, subsequently leading to a decrease in the number of immune cells produced by it.
Infectious diseases require an adequate immune response from the thymus. At the same time, the thymus is a target for many pathogens that enter the organ through the bloodstream, disrupting its structure and distorting the maturation of immune cells.
For example, in 2021, German scientists found that the influenza A virus causes thymus atrophy, increased death of immature immune cells, and decreased diversity. Bacterial infections also affect the thymus gland just as destructively. In 2020, Chinese scientists demonstrated this in a streptococcal disease.
In 2021, another group of Chinese scientists summarized the accumulated knowledge and published a review of infections that cause thymus atrophy. The most common viral diseases are influenza A, herpes, hepatitis C, and HIV. Many mechanisms that cause thymus atrophy are associated with bacterial infections: salmonellosis, E. coli infection, streptococci and staphylococci, and mycobacteria. Much of the review is devoted to parasitic and fungal infections that negatively affect the thymus, releasing toxins.
Chemo- and radiation therapy have a catastrophic effect on the thymus. These methods are used in cancer treatment or before bone marrow transplantation. During therapy, not only tumor cells die, but also thymus cells, peripheral immune cells, and the thymus microenvironment. Chemotherapy significantly worsens the function of the thymus and causes its atrophy – it reduces the lobules of the entire organ and reduces the number and variety of immune cells developing in it.
In 2016, Chinese scientists published a clinical study of 54 adults who received chemotherapy for treating lymphoma. With the help of serial computed tomography, scientists observed the rapid degradation of the organ and its subsequent 12-month recovery. Scientists noted that in young people, the thymus retained its ability to regenerate better than in older adults.
Thymus epithelial cells are resistant to radiation exposure. However, as Swiss scientists showed in 2016, this resistance is significantly reduced during hypoxia (lack of oxygen).
During bone marrow transplantation, the transplant’s immune cells perceive the host’s thymus as a foreign organism and seek to destroy it, causing tissue destruction. The process is exacerbated by interferon-gamma production by donor cells, which accelerates the programmed death of the host’s thymocytes. This mechanism was demonstrated in mice by Swiss scientists in 2007.
Chronic Injury
A decrease in the immune system’s functions characterizes the body’s aging. The main reason for this decrease is the degradation of the thymus: a reduction in its size, deterioration in structure, and replacement of organ tissues with adipose and connective tissues. As a result, immune cells’ maturation is disrupted, their diversity decreases, and vaccination becomes less effective. Thus, in the body of older people, aging immune memory cells accumulate, which leads to chronic inflammation. The thymus undergoes constant age-related aging, regardless of its ability to rejuvenate.
In 2003, Australian scientists calculated that the number of maturing immune cells in the thymus halved on average every 16 years. According to this calculation, by age 55, only 5% of the body’s immune cells leave the thymus gland.
In 2020, scientists from the University of North Texas (USA) published a review of studies on the relationship between age-related degradation of the thymus gland, immune aging and chronic inflammation. Scientists have shown the decisive role of these processes in the progression of age-related neurological, cardiovascular and oncological diseases.
Thymus regeneration strategies
Thymus epithelial cell regeneration
Thymus epithelial cells are critical for the normal functioning of the organ and its rejuvenation. Thymus regeneration research is focused on hormonal therapy and treatment with particular proteins produced by the thymus gland.
The keratinocyte growth factor promotes the reproduction of thymic epithelial cells and protects them from damage.
In 2007, an international team of scientists published a study that describes the mechanisms and timing of thymus rejuvenation. Within two weeks of using the keratinocyte growth factor, the number of thymic epithelial cells returned to normal and the microenvironment of maturing immune cells was restored.
In 2017, another international team of scientists discovered that the antitumor protein p53 activates signals from keratinocyte growth factor to thymic epithelial cells. This protein activates the genes necessary for the maturation of immune cells. In mice lacking this protein, thymic regeneration occurs with defects.
In 2007, American scientists investigated the preparation of recombinant human keratinocyte growth factor for the treatment of cancer patients. 212 participants with blood cancer developed oral mucositis after high doses of chemotherapy and radiotherapy. The keratinocyte growth factor accelerated the treatment of mucositis by about 30%.
Interleukin-22 (IL-22) is critical for the recovery of the thymus after injury.
In 2017, American scientists showed the results of the effect of IL-22 on thymus regeneration after bone marrow transplantation. The transplant caused degradation of the thymus epithelium. At the same time, there was a deficiency of IL-22. The introduction of the drug IL-22 accelerated the regeneration of the thymus. The experiments were carried out on mice.
In 2021, Chinese scientists published the results of a clinical study in which they observed the dynamics of interleukin-22 in patients who underwent bone marrow transplantation. An increase in the content of IL-22 in the blood correlated with the number of mature immune cells. Also, patients with high levels of IL-22 had fewer complications when the graft suppressed the host’s immune system.
Growth hormone stimulates the growth of all body tissues, including the thymus.
In 2016, American scientists showed that plasma levels of growth hormone decrease with age.
In a 2008 clinical trial, growth hormone reversed thymic atrophy in treating patients with HIV. An increase in the number of immune cells in the blood accompanied the process.
Another 2019 clinical study involved healthy men aged 51 to 65 years. That is the first human study to reverse immune aging and reduce epigenetic age, the characteristics of DNA that determine the biological age of an organ, tissue, or individual cell. Scientists at leading US universities used recombinant human growth hormone, administered subcutaneously to participants every other day. Participants also received the following:
- Metformin to prevent diabetes, as growth hormone increases the risk of developing diabetes;
- Prasterone to compensate for the age-related decrease in the adrenal hormone level.
Vitamin D and zinc were used as food supplements.
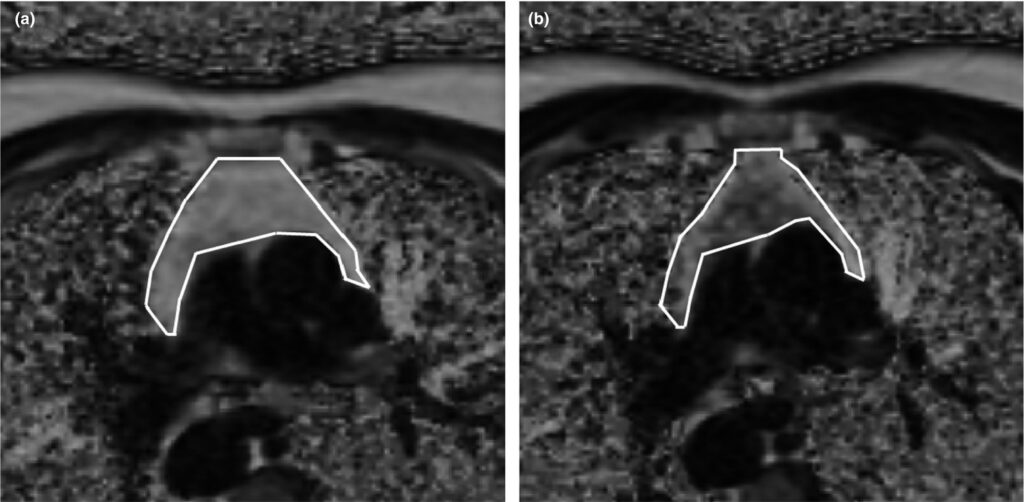
As a result, scientists observed a qualitative improvement in the density of the thymus – the replacement of adipose tissue with functional cells. On the MRI results, adipose tissue cells are marked in light, and functional cells are marked in dark:
Image Source: https://onlinelibrary.wiley.com/doi/10.1111/acel.13028
Scientists observed a similar regeneration process in the bone marrow. The participants also increased the diversity and number of critically critical immune cells. Participants’ epigenetic age regression averaged 2.5 years over the 12 months of the study. The effect persisted for six months after the end of the drugs and supplements.
Recovery of thymocytes
A decrease in the level of sex hormones reduces the thymus gland’s degradation rate. During puberty, levels of testosterone (in men) and estrogen (in women) increase significantly. These hormones cause the programmed death of thymocytes, and estrogen (estradiol) additionally stimulates thymus atrophy. However, this mechanism is not fully understood.
In 2015, an international team of scientists demonstrated that surgical destruction of the hypothalamic-pituitary-gonadal axis contributes to the growth of the thymus and an increase in the diversity of immune cells. This endocrine complex regulates the production of sex hormones.
Similar results were obtained in 2005 by Australian scientists in a study of patients with prostate cancer. It was observed that after androgen blockade, the number and diversity of immune cells increased in patients.
Injections of progenitor cells increase the number of immune cells maturing in the thymus.
As established in 2009 by Canadian scientists, progenitor cells of human immune cells successfully take root in the thymus of immunodeficient mice. The procedure helps to restore their immunity.
This direction was continued by Japanese scientists in 2013. In the lab, scientists reprogrammed stem cells to mimic aging immune cells. As a result, young immune cells capable of intensive reproduction were obtained. Experts note that immature cells had elongated telomeres – the end sections of chromosomes that protect DNA from damage during cell division.
Conclusions
The thymus is an essential organ of the immune system. Under the influence of external factors, it degrades and then recovers. However, with age, its ability to regenerate deteriorates. Natural and artificial glucocorticoids, diseases, chemotherapy and radiation therapy, promote degradation. Most thymus regeneration strategies are under investigation in animal and cell models. The most promising directions are already being tested in small groups of patients and healthy volunteers.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary:
References
- Dynamic of plasma IL-22 level is an indicator of thymic output after allogeneic hematopoietic cell transplantation
- Growth hormone resurrects adult human thymus during HIV-1 infection
- Reversal of epigenetic aging and immunosenescent trends in humans
- Role of Bone Marrow Maturity, Insulin-Like Growth Factor 1 Receptor, and Forkhead Box Protein N1 in Thymic Involution and Rejuvenation
- Sex steroid ablation: an immunoregenerative strategy for immunocompromised patients
- Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells
- Generation of Rejuvenated Antigen-Specific T Cells by Reprogramming to Pluripotency and Redifferentiation
- Activation of Thymic Regeneration in Mice and Humans following Androgen Blockade
- Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations
- Dexamethasone-induced apoptosis of thymocytes: role of glucocorticoid receptor–associated Src kinase and caspase-8 activation
- Improvement of glucocorticoid-impaired thymus function by dihydromyricetin via up-regulation of PPARγ-associated fatty acid metabolism
- Influenza A virus-induced thymus atrophy differentially affects dynamics of conventional and regulatory T-cell development in mice
- Streptococcus suis Serotype 2 Infection Causes Host Immunomodulation through Induction of Thymic Atrophy
- Infection-Associated Thymic Atrophy
- Thymic hyperplasia after chemotherapy in adults with mature B cell lymphoma and its influence on thymic output and CD4+ T cells repopulation
- Differential Response of Mouse Thymic Epithelial Cell Types to Ionizing Radiation-Induced DNA Damage
- Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation
- Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty
- Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging
- Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells
- Thymic epithelial cells require p53 to support their long-term function in thymopoiesis in mice
- Palifermin for Oral Mucositis after Intensive Therapy for Hematologic Cancers
- Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft-versus-host disease