The body can destroy pathogens that it has encountered before. This ability is called acquired immunity – it can kill virus-infected cells and generate protective immunological memory. It is the basis of vaccination strategies.
T cell and B cell responses are essential for fighting viruses and developing immunological memory. Once in the body’s cells, viruses control cellular mechanisms to create new viral particles and spread them to healthy cells. Viral infections cause innate immune responses, such as inflammation. These reactions activate the acquired immunity. Dendritic cells capture viral proteins and particles and transport them to the lymphoid organs – the spleen and lymph nodes. There, viral proteins and particles are recognized by T and B cells. The cellular (T cell) and humoral (B cell) branches of acquired immunity work together to provide virus-specific protection.
A mechanism to recognize and eliminate the virus
Both T cell recognition of viral peptides and B cell recognition of viral proteins begin in lymphoid tissues, where dendritic cells present peptides to T cells and B cells select viral proteins. Together, they initiate the development of effector cells to eliminate the virus.
The magnitude of the T and B cells responses is determined by:
- the pathogenicity of the virus;
- degree of inflammation;
- the frequency of virus-specific T and B cells;
- the kinetics of virus replication.
CD8+ T cells (T-killers) differentiate into effector cells, limiting the virus’s replication, destroying infected cells. CD4+ T cells (T-helpers) differentiate into effector cells that inhibit virus replication and support CD8+ T cells and B cell differentiation. Both CD8+ and CD4+ T cells produce the antiviral and immunomodulatory peptide interferon-gamma. To trigger an antiviral response, effector T cells can enter the bloodstream and travel to infection sites.
CD4+ T cells also differentiate into follicular T helper cells (ТFH), which are essential for developing antibody-producing B cells and supporting memory B cells’ development. TFH is crucial for the formation of germ centers in the follicles of peripheral lymphoid organs.
Antibodies can neutralize viruses, preventing them from entering cells or contributing to the death of infected cells. As a result of the coordinated interaction of innate and acquired immunity, peak T – and B-cell responses lead to decreased viral load and a decrease in inflammation, often within one week after infection.
T and B memory cells
After the virus is removed, most effector T and B cells die. The preserved T cells become memory T cells. Preserved B cells either generate long-lived plasma cells that produce antibodies, or they become memory B cells. Memory cells can respond quickly to reinfection.
SARS-CoV-2-specific CD4+ and CD8+ T cells are found in the bloodstream in both actively infected and recovered patients, indicating the potential for the development of protective cellular immunity.
Memory T-cells can be:
- effector – responsive with high titers of the virus;
- central – serving as a reservoir for protection during future reinfections.
T cells located in the foci of infection can become tissue-resident memory T cells (TRM).
What determines the effectiveness of the acquired immune response?
When the acquired immune responses to infection are suboptimal, or the virus has developed means to evade immune responses (including suppressing immunity by the virus), chronic infection or systemic disease and death may occur, especially in at-risk individuals. The severity of the illness in SARS-CoV-2 coronavirus varies. Therefore, it is necessary to understand what factors determine the acquired immune response’s effectiveness and long-term protective immunity, especially in vulnerable populations: the elderly and people with weakened immunity.
A critical factor in eliminating viral infections is the sustained, highly effective antiviral responses of T and B cells. Naive T and B cells have a variety of viral recognition receptors on the surface so that they can recognize a wide range of viruses. During infection, T cells with the most suitable T cell receptors are selected – they have a more remarkable ability to bind viral peptides. B-cell receptors’ affinity for viral proteins continues to increase during infection through somatic hypermutation and clonal expansion. Somatic hypermutation is a mechanism that provides a variety of B-cell receptors and antibodies. Thanks to it, the immune system can recognize more different pathogens. Clonal expansion is the process of B-cell division, in which multiple copies appear from a single cell.
B cells switch from the early synthesis of class M antibodies (IgM) to the synthesis of IgG and IgA antibodies with higher affinity. These antibodies are found in the serum of recovering patients with COVID-19.
The magnitude of early antibody responses may indicate the severity of the infection, as higher IgM and IgG antibody titers are associated with more severe disease. The total serum antibody titers for SARS-CoV-2 decrease after the active infection is cured with other viral infections. However, long-lived memory B cells can persist and produce circulating virus-neutralizing antibodies. A recent study showed that 6 to 8 months after the onset of COVID-19, neutralizing antibodies against SARS-CoV-2 were detected in 90% of recovered patients. It is also essential to track and compare the duration of the memory B cell response to natural SARS-CoV-2 infection and the vaccine.
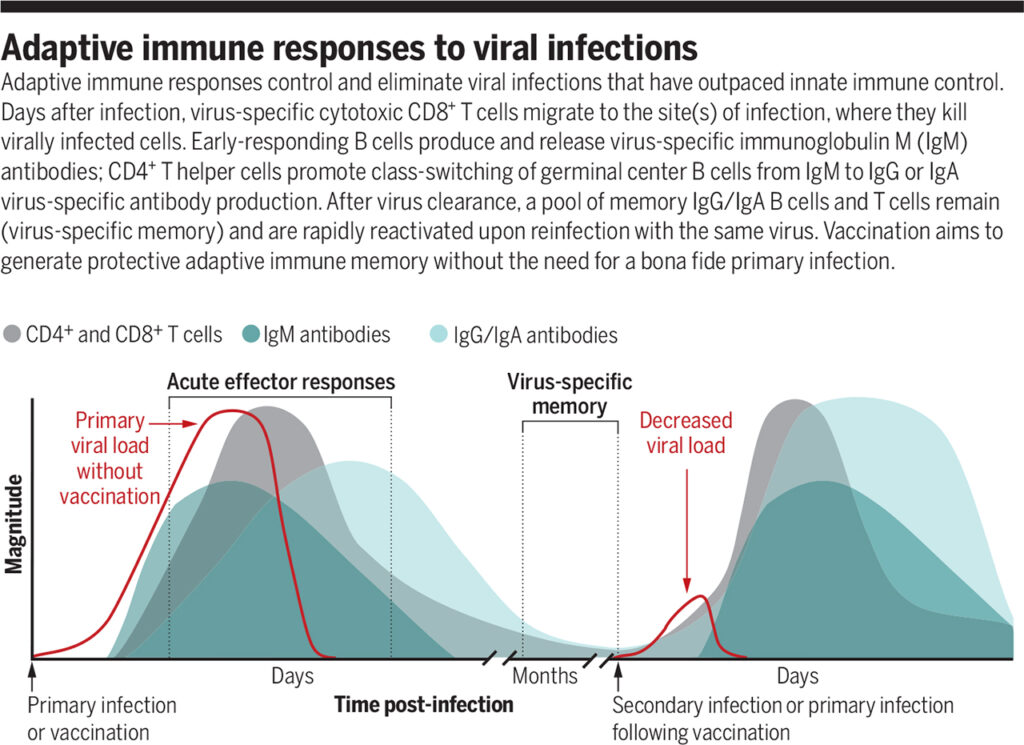
Acquired immune response to viral infections
Source: https://www.science.org/doi/full/10.1126/science.abf6446
The acquired immune response controls and eliminates viral infections that have outpaced innate immune power. A few days after illness, virus-specific cytotoxic CD8+ T cells migrate to the infection site, killing the virus-infected cells. B cells with an early response produce virus-specific IgM antibodies. CD4+ T helper cells promote the switching of the germ center class of B cells from IgM to IgG or the production of virus-specific IgA antibodies. After the virus is removed, the IgG / IgA memory B cells and the memory T cells are preserved. They are quickly reactivated when re-infected with the same virus. Vaccination aims to form a protective acquired immune memory without the need for a genuine primary infection.
Cross-reactive T cells and antibodies
Previous encounters with viruses may influence the responses of the acquired immunity. For example, TRM cells located in the lungs can recognize influenza viruses’ internal structural proteins and reduce infection severity with new influenza viruses. T cells that are reactive to SARS-CoV-2 proteins have been found in individuals previously infected with SARS-CoV and in healthy individuals who have not been exposed to these coronaviruses. These results show that memory T cells are generated when infected with other human coronaviruses (HCoV), including the common cold coronaviruses. The IgG antibodies of patients with COVID-19 were found to react strongly to hepatitis C virus proteins. In contrast, SARS-CoV-2-reactive antibodies specific to HCoV proteins have been found in the sera of people who have not had COVID-19 – these can be broadly neutralizing antibodies.
It is not yet clear whether existing SARS-CoV-2-reactive T and B cells can contribute to improved disease outcomes. If they improve the disease’s outcome, it may be desirable to increase such T and B cell populations through vaccination.
What is the danger of excessive activation of T-cells?
It is crucial to monitor the extent of infection and inflammation caused by viruses, including SARS-CoV-2. Exaggerated innate immune responses, such as hyperinflammation (elevated levels of interleukin-6 (IL-6) and C-reactive protein), can contribute to T-lymphocytes’ pronounced activation, further increasing inflammation and the severity of the disease. Extensive T-cell activation during COVID-19 can lead to lymphopenia (a decrease in the number of lymphocytes), altered differentiation, and T-cell function loss. This can slow down a viral clearance, prolong infection, and increase morbidity.
Factors predicting the severity of the disease
It remains unclear why some infected people have no symptoms, while others have severe COVID-19. However, severe COVID-19 is associated with:
- Violation of the innate immune responses of interferon type I.
- Cytomegalovirus (CMV) and herpes simplex virus 1 (HSV-1).
- Male gender. Men hospitalized with COVID-19 have a more robust antibody response and increased disease severity compared to women.
- Age > 65 years. During acute SARS-CoV-2 infection, older people were more likely to have unregulated responses of acquired immunity than younger people.
- Early antibody profile. American scientists have found that early differences in the antibody profiles of spike protein (S) and nucleocapsid protein (N) of the SARS-CoV-2 coronavirus predict a mild or severe course of COVID-19. S-antibodies were elevated in recovering individuals, while N-antibodies were elevated in deceased patients. In contrast, sustained T-cell responses to S, N, and membrane protein (M) were not associated with better recovery in critically ill COVID-19 patients.
- The composition of the intestinal microbiome. American researchers have shown that an increase in the number of Enterococcus faecalis intestinal bacteria is the main predictor of severe COVID-19.
The ability to identify predictive factors will be increased by including asymptomatic individuals infected with SARS-CoV-2.
Dangerous reactions of acquired immunity to SARS-CoV-2
German scientists found that highly mutated B-cell receptors and reduced clonal expansion were associated with more severe clinical outcomes in actively infected patients. Meanwhile, a larger pool of virus-specific naive B cells in patients correlated with developing a more effective antiviral immune response.
Enhanced TH1 differentiation, loss of TFH, and the associated lack of germ center formation were recently detected in postmortem analysis of lymph nodes and spleen in patients who died within 10 days of the COVID-19 respiratory symptoms onset. Altered proportions of peripheral blood CD4+ T cell subpopulations, CD8+ T cell activation status, and B cell activation status were also observed in patients with severe COVID-19. In-depth analysis of about 200 immune parameters showed that the severity of the disease, including severe inflammation and organ failure, correlated with general lymphopenia, while the remaining lymphocytes-CD4+ and CD8+ effector T cells, TFH cells, and short-lived plasma cells that produce antibodies-were highly activated and potentially hyperactive.
Conclusion
The main factors underlying the various reactions to SARS-CoV-2 remain unclear. One potential area for future research is to assess whether prior infection with other respiratory diseases or immune-mediated conditions such as asthma may increase susceptibility to the SARS-CoV-2 coronavirus.
Most studies evaluating the responses of acquired immunity to the SARS-CoV-2 coronavirus analyzed infected adults. Such studies on children are more limited. Understanding the differences in acquired immune responses between children and adults is an essential issue with SARS-CoV-2 because children are less at risk of severe respiratory complications than adults, but they can develop the life-threatening multisystem inflammatory syndrome. It may reflect an altered recognition of the virus by the immune system in children. A recent study comparing antibody specificity in adults and children with SARS-CoV-2 infection showed reduced antibody diversity in children and a distorted response to IgG S-antibodies.
Studies evaluating the innate and acquired immune response, clarifying the interaction between T and B cells, and determining their relationship to protection against coronavirus will help develop strategies to create and strengthen antiviral immunity.