Content
- Immune cells produce interferon in response to infection with the bacterium Helicobacter
- Helicobacter defends itself against the immune system by taking away cholesterol from the epithelial cells of the stomach
- Cholesterol injection fools Helicobacter pylori and restores protective interferon response but increases inflammation
- Helicobacter inhibits interferon synthesis boosted by probiotics
- Treatment of Helicobacter pylori with recombinant interferon-α2b
- Interferon-gamma contributes to protection against Helicobacter pylori
- Conclusion
- References
Helicobacter pylori is a bacterium that is the leading cause of chronic gastritis, peptic ulcer, and stomach cancer. Approximately half of the world’s population is infected with this bacterium, which has evolved with humans over the past 30,000 years. During this time, the bacterium has developed numerous defense mechanisms against the immune system, including blocking interferon signals.
Immune Cells Produce Interferon In Response to Helicobacter
This mechanism was studied in detail by an international team of scientists in 2022:
- First, immune cell monocytes engulf the bacteria. In destroying bacteria, monocytes detect foreign genetic information – RNA – and stimulate cells to produce type I interferon (IFN-1).
- Interferon molecules attract other immune cells – lymphocytes, dendritic cells – to the focus of infection.
- IFN-1 stimulates the production of proteins that cause an inflammatory response to cope with the bacterium.
Helicobacter Defends Itself against The Immune System by Taking Away Cholesterol From The Stomach Epithelial Cells
The Helicobacter bacterium deprives the stomach’s epithelial cells of the opportunity to receive the interferon signal and produces antibacterial proteins to protect against severe inflammation and avoid a perceptible immune system reaction.
This defense mechanism of bacteria was discovered by German scientists in 2018. Scientists have found that Helicobacter extracts cholesterol from the membranes of the stomach’s epithelial cells. As a result, the geometry of membrane proteins that trap IFN molecules is disrupted. The bacterium uses cholesterol for reproduction – its cell membrane also consists of cholesterol, which it cannot produce itself.
As a result of the work of the protective mechanism, an area of reduced inflammation, a smaller number of immune cells and antibacterial defense proteins is formed in the lesion. Bacteria live in this area. Moreover, in regions neighboring with healthy epithelium, on the contrary, there is increased inflammation and a high concentration of pro-inflammatory protein molecules and immune cells.
The Introduction of Cholesterol Increases Inflammation but Deceives Helicobacter Pylori and Restores The Protective Response Of Interferon
The scientists continued to investigate the effects of cholesterol and injected water-soluble cholesterol into the infected areas. As a result, the bacteria switched to this external source of cholesterol, and the membranes of the stomach’s epithelial cells were restored, became susceptible to interferon molecules and began to produce highly effective proteins that destroy Helicobacter.
An earlier study by the same scientists demonstrated how a cholesterol-rich diet could help reduce Helicobacter pylori. The scientists kept two groups of mice infected with the bacterium on different diets. One group ate only cholesterol-free foods. The second group was 2% cholesterol content. After 25 weeks, cholesterol-fed mice had 95% lower H. pylori levels than mice on a cholesterol-free diet. However, mice in the cholesterol group had significantly higher levels of inflammation. Scientists explain this effect by attracting other immune cells to the affected areas and the production of antibacterial defense proteins, which, on the one hand, significantly reduce the number of Helicobacter pylori but, on the other hand, increase inflammation.
In the same study, scientists observed Helicobacter pylori migrate to areas with higher cholesterol concentrations. Test tubes connected were used for the experiment. In one – a culture of bacteria. In others – a solution of cholesterol in different concentrations. It has been found that Helicobacter pylori picks up cholesterol concentrations 250 times lower than in the blood and travels to the source.
Based on these results, scientists recommend a low-cholesterol diet. In their decision, the experts refer to a study by Turkish scientists who found severe forms of gastritis in people with high levels of low-density lipoproteins and infected with Helicobacter:
Image source: https://link.springer.com/article/10.1007/s10620-008-0391-y
Helicobacter Inhibits Interferon Synthesis Boosted by Probiotics
Another mechanism for protecting Helicobacter from the immune system is associated with synthesizing interferon molecules and not with signaling, as in the previous case.
Danish scientists found that probiotic bacteria stimulate immune cells to produce interferon-beta (IFN-β) and analyzed 43 strains of probiotics to find the most effective strain. The article “How to Choose the Right Probiotic” details the study.
However, Helicobacter pylori produce a unique toxin VacA, which blocks the production of IFN-β stimulated by probiotics and thus prevents the launch of adaptive immunity. This mechanism was discovered in 2013 by an international group of scientists. Read more about the VacA toxin in the article “How Helicobacter Interferes with Probiotics to Stimulate The Synthesis of IFN-β”.
Scientists were interested in conflicting data from earlier clinical studies. In some studies, probiotic bacteria reduced the number of Helicobacter pylori and reduced the side effects of standard antibiotic treatment. However, another study in 62 patients with peptic ulcers and ulcerative scars found no positive impact of probiotics.
As a result of the study, several important discoveries were made:
- Stimulation of immune cells by probiotics increases the synthesis of IFN-β by 400 times.
- Adding Helicobacter pylori to probiotic bacteria at a concentration of 1:1 blocks the production of IFN-β.
- The negative effect of Helicobacter is leveled by the pre-treatment of immune cells with a probiotic for 3.5 hours.
- Higher concentrations of probiotic bacteria slightly increase IFN-β synthesis. Moreover, only starting from a concentration of 16: 1, the negative effect of Helicobacter pylori on the production of interferon-beta becomes insignificant.
- Blocking the synthesis of IFN-β is not associated with the action of individual Helicobacter bacteria but with their production of particular substances. VacA toxin, produced by Helicobacter pylori, contributes to the formation of acidic vacuoles in cells and the disruption of the structure of cell membranes. This toxin was discovered in 2000 as a factor that impairs the protective properties of the mucus lining the stomach. In the present study, the scientists tested their hypothesis using a genetically modified Helicobacter bacteria that cannot produce the VacA toxin. The genetically modified bacteria did not affect the synthesis of interferon-beta stimulated by the probiotic.
Treatment of Helicobacter pylori with Recombinant Interferon-α2b
Helicobacter inhibits the synthesis of interferon and impairs the transmission of its signals. Therefore, interferon preparations can be used to treat gastritis and peptic ulcer.
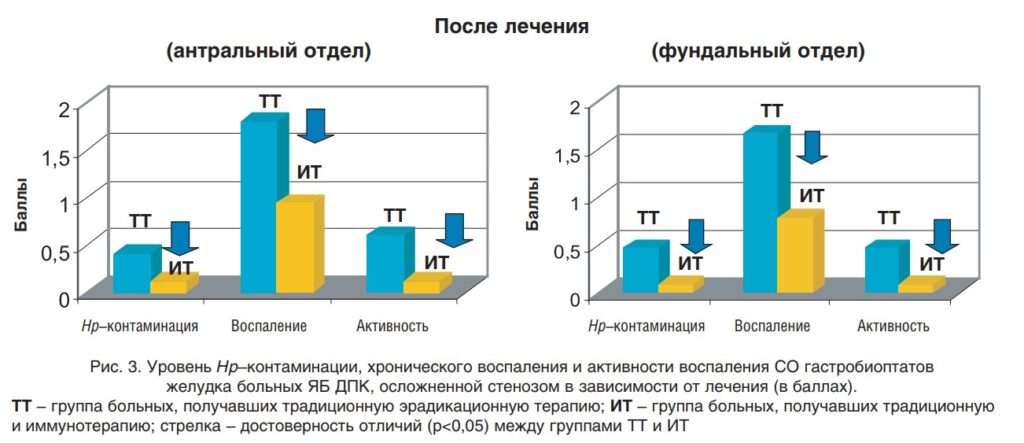
In 2006, scientists from the Russian Center for Functional Surgical Gastroenterology investigated using a systemic preparation of interferon-α2b in postoperative therapy. Scientists have observed the dynamics of inflammation and the number of Helicobacter pylori in traditional treatment and immunotherapy with IFN-α2b.
The study involved 49 people with chronic gastritis and duodenal ulcer. All patients underwent radical duodenoplasty and subsequent restorative treatment.
The study showed that if the systemic drug IFN-α2b is included for immunotherapy in the early postoperative period, then the number of Helicobacter bacteria decreases by about three times in the antrum and the fundus by five times. At the same time, the level of inflammation in both departments is reduced by two times:
Image source:
https://www.rmj.ru/articles/obshchie-stati/Effektivnosty_rekombinantnogo_interferona_a2b_pri_eradikacii_Helicobacter_pylori_u_pacientov_s_osloghnennoy_yazvennoy_boleznyyu_dvenadcatiperstno/
Interferon-gamma Contributes to Protection against Helicobacter Pylori
One of the first studies on the role of interferons in Helicobacter infection was conducted in 1999 by scientists from the Kyoto Medical University (Japan). Experts showed that genetically modified mice, unable to produce interferon-gamma, were 30% more susceptible to infection with Helicobacter pylori than wild-type mice. Moreover, in genetically modified mice, the concentration of bacteria was higher.
However, wild-type mice developed erosions in the gastric epithelium 15 months after infection, and mice unable to produce IFN-γ showed no signs of inflammation. Moreover, in wild-type mice, a mild degree of stomach inflammation appeared after four weeks; the average was after eight weeks.
At the same time, a strain of Helicobacter pylori, which was unable to colonize wild-type mice, successfully infected mice unable to produce interferon-gamma.
The scientists concluded that IFN-γ is vital in protecting against Helicobacter pylori infection. However, at the same time, interferon-gamma stimulates stomach inflammation when the disease has already occurred.
Conclusion
The bacterium Helicobacter blocks interferon signals to protect itself from an immune system response and create safe areas. The protective mechanism is based on the extraction of cholesterol from the membranes of the stomach’s epithelial cells. As a result, the geometry of IFN receptors is disrupted, and cells do not produce antibacterial defense proteins. A cholesterol-rich diet significantly reduces H. pylori levels but increases inflammation and can cause severe gastritis.
Useful article, necessary information? Share it!
Someone will also find it useful and necessary:
References
- Immune evasion strategies used by Helicobacter pylori
- Helicobacter pylori Infection Elicits Type I Interferon Response in Human Monocytes via Toll-Like Receptor 8 Signaling
- Helicobacter pylori Depletes Cholesterol in Gastric Glands to Prevent Interferon Gamma Signaling and Escape the Inflammatory Response
- Cholesterol glucosylation promotes immune evasion by Helicobacter pylori
- The Relationship Between Updated Sydney System Score and LDL Cholesterol Levels in Patients Infected with Helicobacter pylori
- Эффективность рекомбинантного интерферона a2b при эрадикации Helicobacter pylori у пациентов с осложненной язвенной болезнью двенадцатиперстной кишки
- Role of Gamma Interferon in Helicobacter pylori-Induced Gastric Inflammatory Responses in a Mouse Model